在前一期介绍中, 同学们已经了解了AP化学考试的选择题。今天我们将更加深入的了解AP化学考试的第二种题型—简答题。在之前的介绍中,我们已经知道AP化学考试包含【3长+4短】7道简答题。
题型1:Short Free Response Questions 短简答题
真题示例
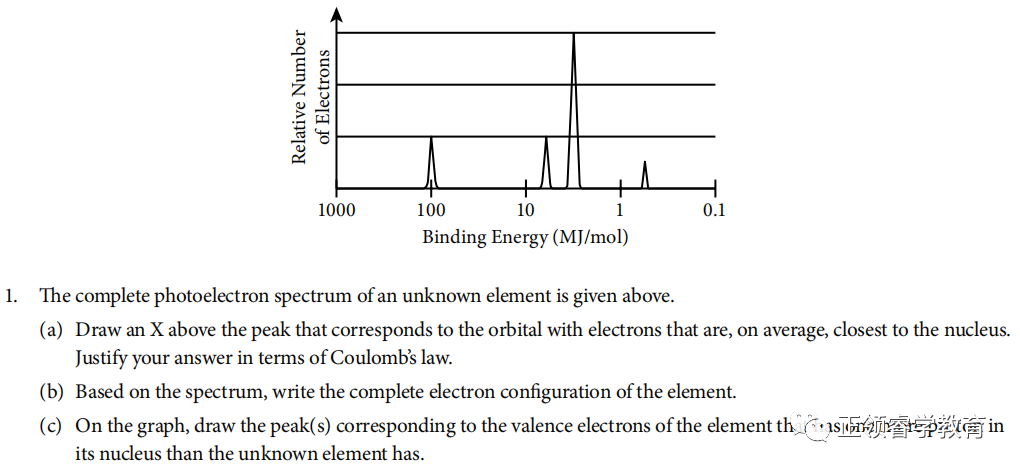
资料来源:College Board
解析:
(a)
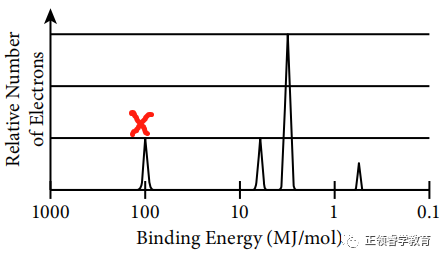
Electrons closest to the nucleus have the greatest binding energy because according to Coulomb’s Law, the strength of attraction between the charges is greatest when the distance between them is the least.
(b) Based on spectrum, the electronic configuration is 1s22s22p63s1.
(c)
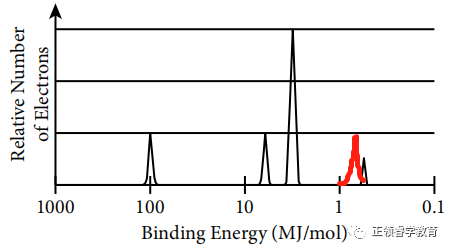
One more proton in nucleus leads to stronger attraction to valence electrons so the binding energy is greater (to the left), and there are two valence electrons in this element; so the height of peak is also twice as that of the unknown element.
题型2:Long Free Response Questions 长简答题
真题示例:
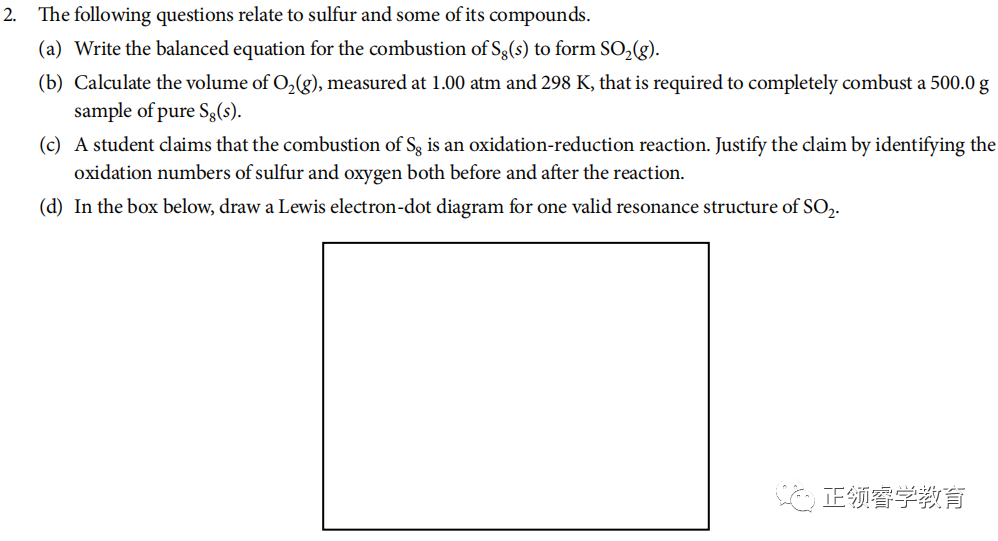
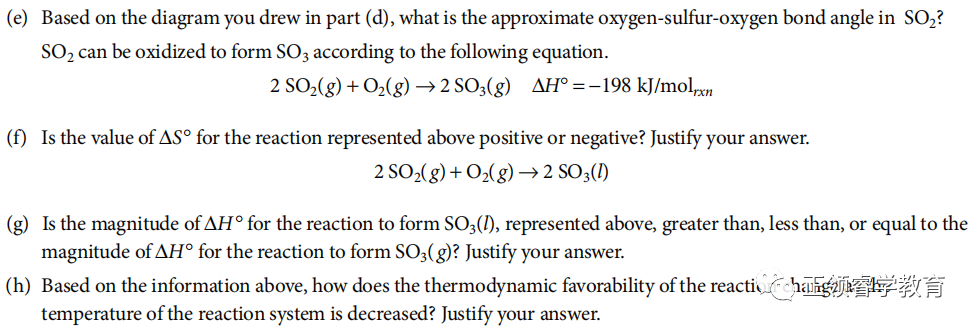
资料来源:College Board
解析:
(a) S8(g) + 8 O2(s) → 8 SO2(g)
(b) Use chemical equation to carry out calculations:
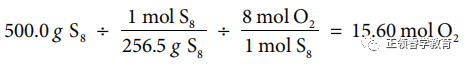
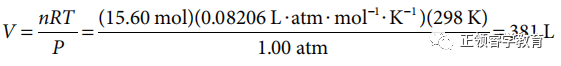
(c) Oxidation numbers before the reaction: S = 0, O = 0
Oxidation numbers after the reaction: S = +4, O = −2
(d)
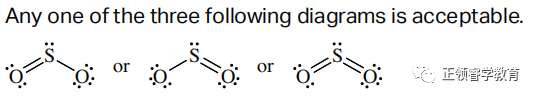
(e) 120o.
(f) Negative, because the reactants are three moles of gas, but the products are only two moles of gas.
(g) Greater, because the enthalpy of SO3 (l) is lower than the enthalpy of SO3 (g) (by an amount equal to the enthalpy of vaporization of SO3 (l)), which makes the enthalpy change of the reaction greater.
(h) ΔG= ΔH− TΔS. Assuming that both ΔH and ΔS remain constant, as the value of T decreases, the smaller in value the term TΔS becomes, making the term ΔH−TΔS more negative. Thus ΔG becomes more negative, which increases the thermodynamic favorability of the reaction.