爱德思化学2023年1月18日场unit6实验题考试相对于往年的真题,没有太多意外,唯一不同就是第二题原本应该是“有机检测”,这次变成了“实验操作”题。所以这次考试就没有“有机检测”题了。可以说是意料之内吧,因为这几年爱德思都在改革与创新。
第一题:无机检测。
第二题:无机+有机实验题
第三题:无机实验题
第四题:有机实验题
接下来对答案吧(仅供参考,不代表官方mark scheme)。
(b)(i) 问加了sodium hydroxide产生绿色的沉淀green precipitate,但是turned brownon standing 的是:Fe(OH)2
Guide对应知识点:
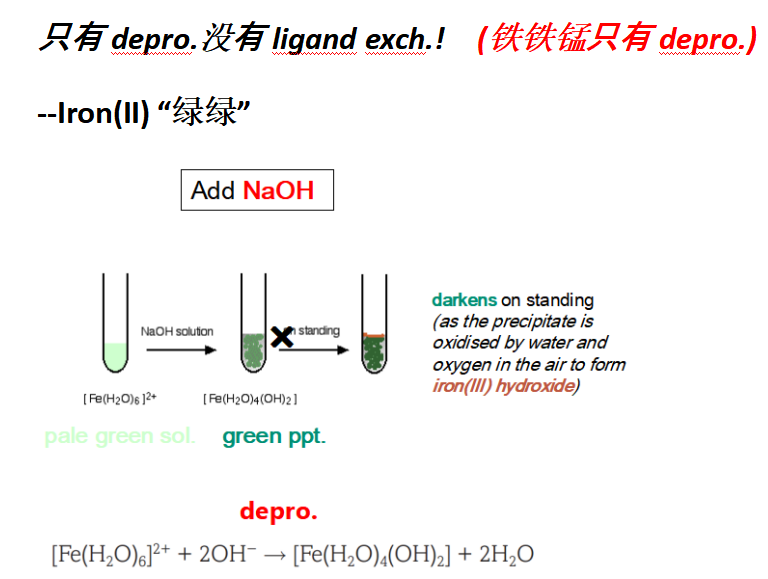
(b)(ii) 问加了sodium hydroxide产生绿色沉淀,然后 dissolved to give a dark green 溶液的是:[Cr(OH)6]3+
Guide对应知识点:
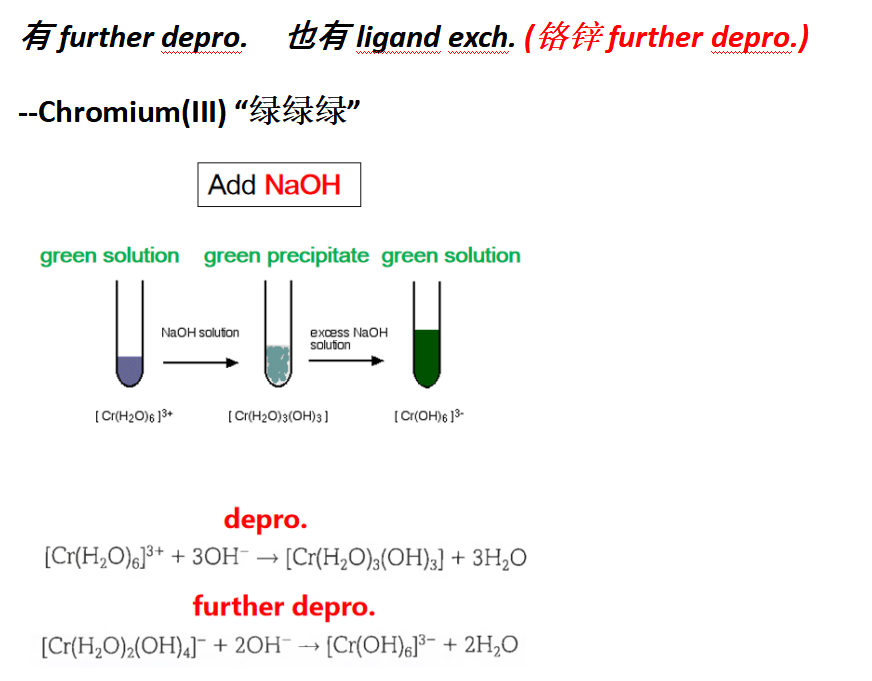
(c)(i) 问加了hydrogen peroxide产生dark green solution turned yellow 的是什么类型反应:oxidation or redox
Guide对应知识点:
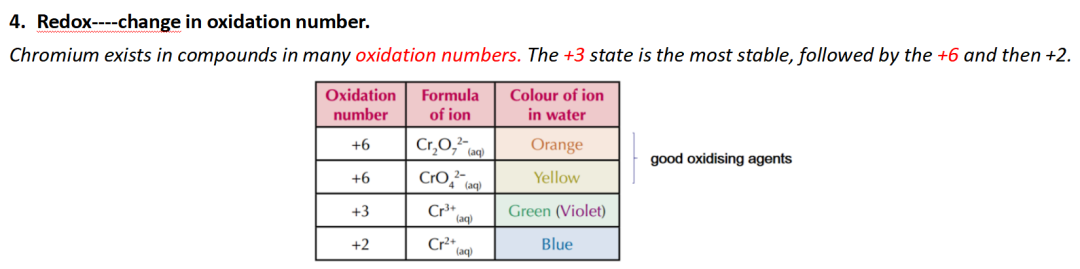
(c)(ii) 问加了酸产生orange colour 的是什么离子:Cr2O72-
Guide对应知识点:
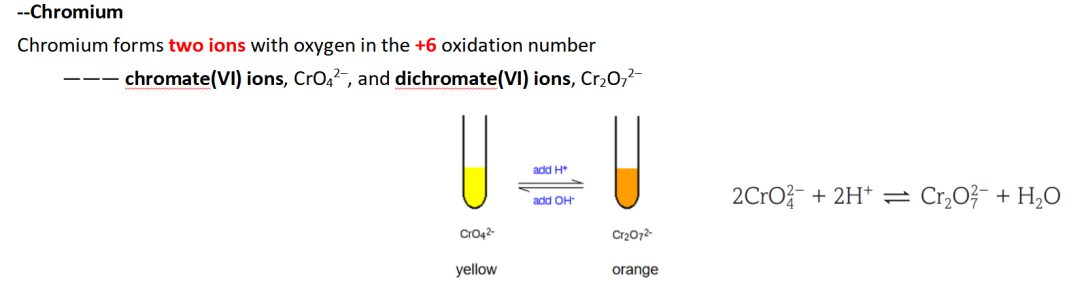
(c)(iii) 问corrosive 要注意什么:Wear gloves
(c)(iv) 描述或者画图展示corrosive hazard:
Guide对应知识点:
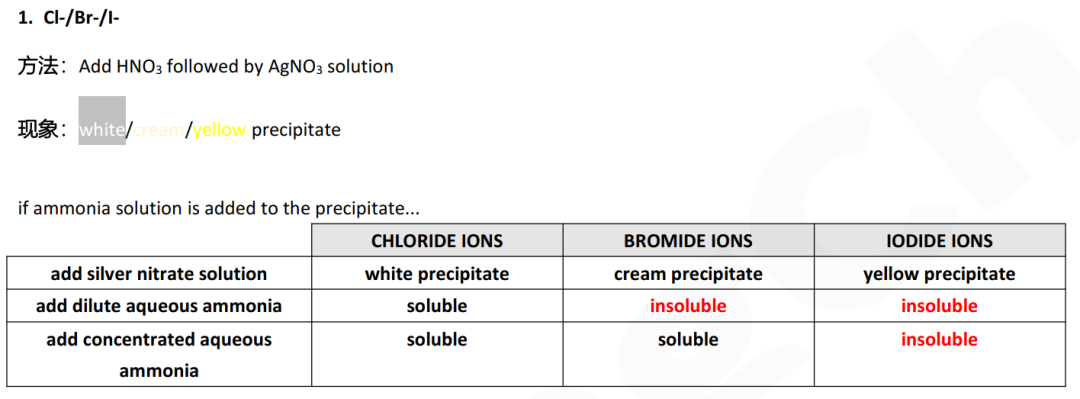
d (i) two anions give white or cream precipitates:Cl- Br-
Guide对应知识点:
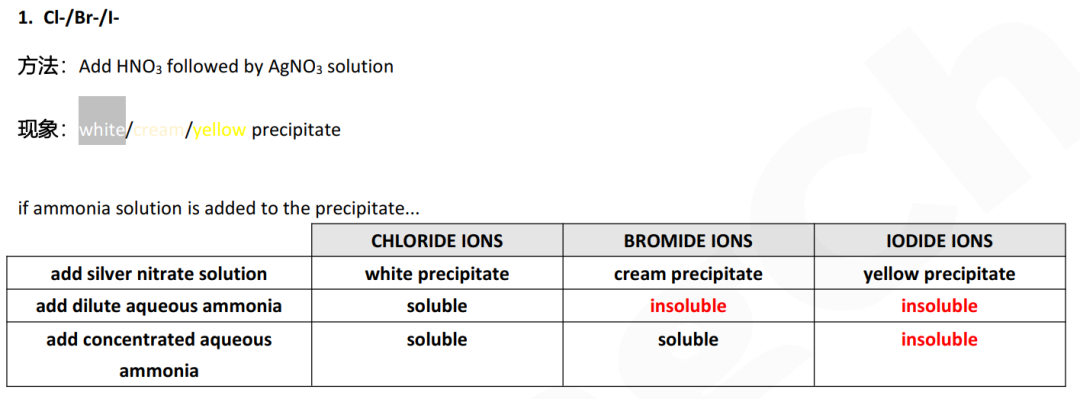
d (ii) two anions give white or cream precipitates:
the precipitate will be insoluble in Br- ionsthe precipitate will be soluble in Cl- ions
Guide对应知识点:
d (iii) 解释为什么加氨水之前precipitate must be separated:
Because the solution A mixture contain HNO3, which will react the NH3(aq) added.
2 a (i) 甲基橙的colour change at the end‑point :
yellow to orange
(ii) 如果Step 4 之后没有立刻titration会有什么影响:
less HClbecause the remain OH- will continue react with(CH3)3CCl, and less OH- left.
(iii) 解释为什么aqueous ethanol会让反应加快:
(CH3)3CCl → (CH3)3C+
the carbocation(CH3)3C+ can be stabilized by aqueous ethanol which contain partially negatively charged oxygen.
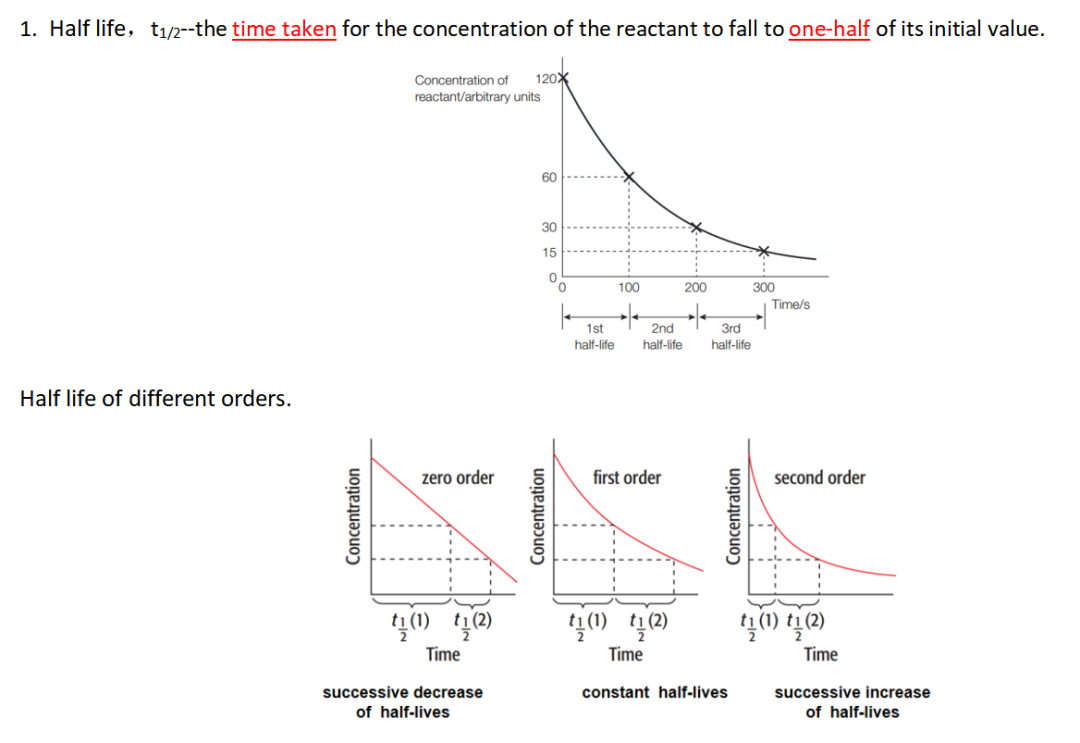
b (i) - (iii) 画图,判断order,确定half-life。
Guide对应知识点:
3 (a) (i) titration 的时候如果不酸化:
formation of manganese(IV) oxide rather than Mn2+
(ii) 酸化为什么不能用hydrochloric acid or nitric acid:
HCl:Cl- can be oxidised by manganate (VII)
HNO3: HNO3 can also oxidise Fe2+
(b) 求Fe2+的百分比:
n(MnO4-)= 0.0200×0.04035=0.000807 mole
n(Fe2+) in 50cm3 = 0.000807×5=0.004035 mole
m(Fe2+) = 0.004035×55.8 = 0.225g
% Fe2+ = 0.225/4.5 = 5.0%
(c) 如果three ways to give the most accurate:
Keep stirring during the titration
Add drop by drop when approaching to the endpoint
Rinse burette each time before using.
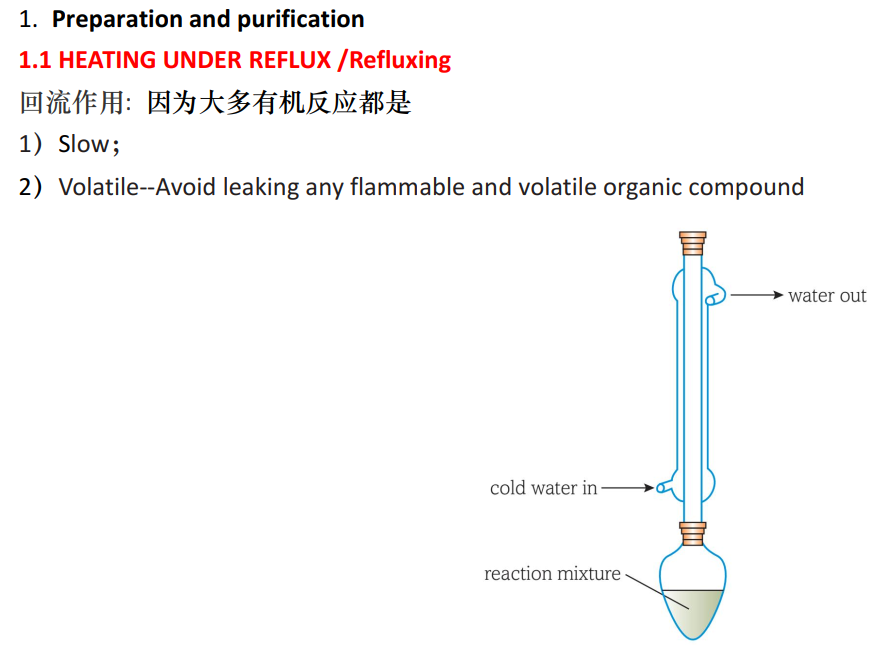
4 (a) 为什么要heat a reaction under reflux。
Guide对应知识点:
(b)Step 4 为什么水要慢慢加?
avoid evaporation of ethanoyl chloride
ethanoyl chloride is volatile and once being stirring, it is easily evaporated.
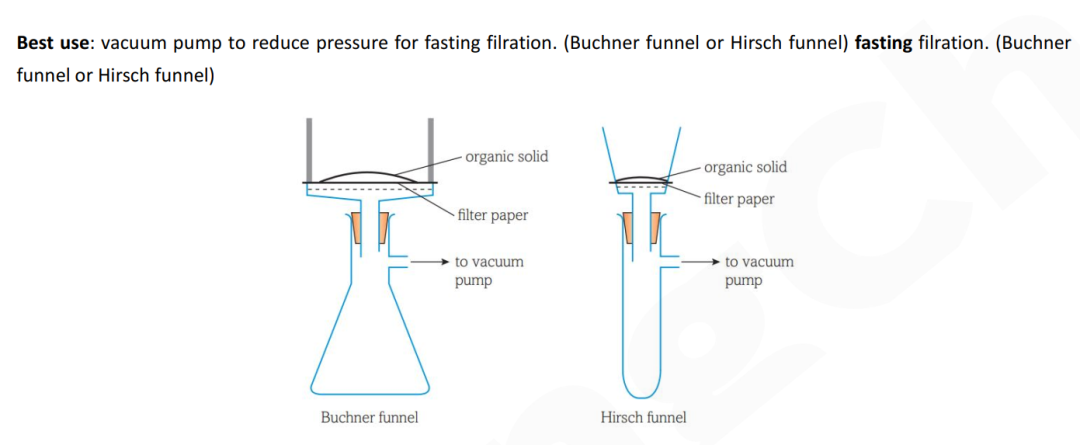
(c)画图:filtration under reduced pressure
Guide对应知识点:
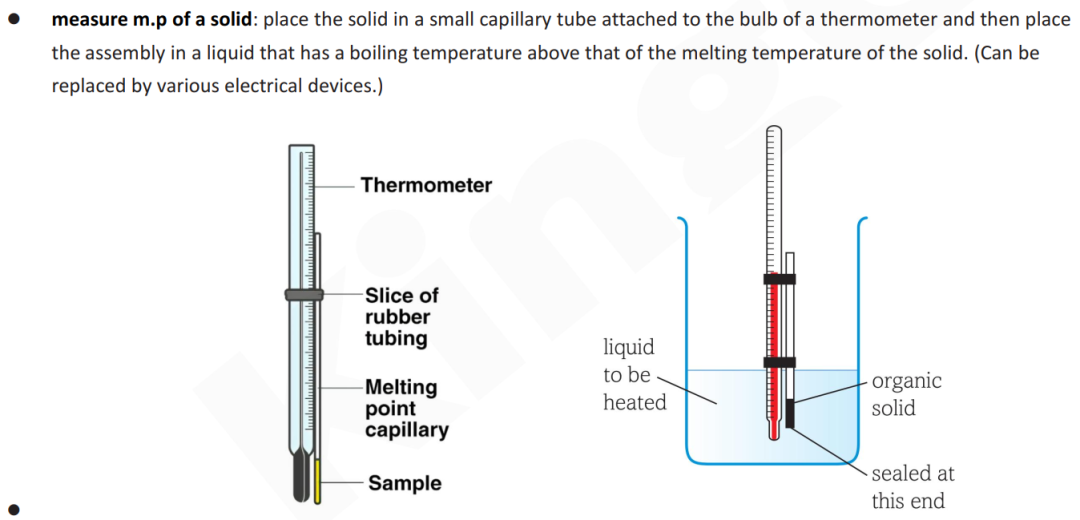
(d)(i) 画图:measure the melting temperature
Guide对应知识点:
(ii)为什么measure the melting temperature 能反映纯度。
Guide对应知识点:

(e)计算哪个过量。
10 × 1.1 = 11g
11÷78.5= 0.14 mole of ethanoyl chloride
5.0 ÷ 137= 0.036 mole of 2‑aminobenzoic acid
0.14>0.036
(f)计算mass of 2‑ethanoylaminobenzoic acid。
Mr(2‑ethanoylaminobenzoic acid)
= 78.5+137-36.5
=179
m(2‑ethanoylaminobenzoic acid)
=179×0.036
=6.444g
6.444×56.7%
=3.65g